Magnesium is a chemical element – a substance that contains only one type of atom. Its official chemical symbol is Mg, and its atomic number is 12, which means that magnesium has 12 protons in it nucleus.
Magnesium compounds were first discovered in a region of Greece known as Magnesia. Some of the first uses were in the form of magnesium sulfate – better known by its common name Epsom salt. Legend says that a farmer in Epsom, England, wanted his cattle to drink water from a local well. The cattle did not like the bitter-tasting water, but the water was useful for healing skin conditions. People still use Epsom salt in their baths to ease sore muscles.
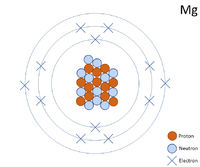
Diagram of the nuclear composition and electron configuration of an atom of magnesium-24 (atomic number: 12), the most common isotope of this element. The nucleus consists of 12 protons (red) and 12 neutrons (blue). 12 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Magnesium dihydroxide is a magnesium hydroxide in which the magnesium atom is bound to two hydroxide groups. It has a role as an antacid and a flame retardant. It has a role as an antacid and a flame retardant. Although it is the eighth most abundant element in the universe and the seventh most abundant element in the earth's crust, magnesium is never found free in nature. Magnesium was first isolated by Sir Humphry Davy, an English chemist, through the electrolysis of a mixture of magnesium oxide (MgO) and mercuric oxide (HgO) in 1808. Name: Magnesium Symbol: Mg Atomic Number: 12 Atomic Mass: 24.305 amu Melting Point: 650.0 °C (923.15 K, 1202.0 °F) Boiling Point: 1107.0 °C (1380.15 K, 2024.6 °F) Number of Protons/Electrons: 12 Number of Neutrons: 12 Classification: Alkaline Earth Crystal Structure: Hexagonal Density @ 293 K: 1.738 g/cm 3 Color: grayish Atomic Structure. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
Alkaline earth metals
Magnesium is grouped with the alkaline earth metals – sometimes referred to as group 2 elements – in the periodic table. The term ‘earth’ was used by early chemists. They initially recognised most of the group 2 earths in their oxide (or compound) forms before they were isolated as elements. They used the term earth to describe non-metallic substances that are stable when heated and not able to be dissolved in water. Alkaline means the oxides are basic or have a pH above 7.
The other elements in this group are beryllium, calcium, strontium, barium and radium. British chemist Humphry Davy used electricity to isolate magnesium and other group 2 elements in 1808.
Currently, we’ve discovered or created 118 elements. If scientists are able to create element 120, it will mostly likely belong to the alkaline earth metal group. Like radium, element 120 will also likely be radioactive.
Flash powder, fireworks and flares
Magnesium is an unusual metal in that it is difficult to ignite when there is a chunk of it, but when it is burning, it is very hard to extinguish. It continues to burn in nitrogen, carbon dioxide and even in water!
On the other hand, magnesium powder and shavings are easy to ignite. When it burns, it gives off a brilliant white light. Magnesium powder was used as flash powder in the early days of photography. Now it is used in fireworks and marine flares. Outdoors enthusiasts often carry small blocks of magnesium – which they shave – to start their fires.
A very useful element
Magnesium is the fourth most common element in the Earth after iron, oxygen and silicon. There’s so much magnesium, we could create four very enormous objects – one the size of Mars and three the size of the Moon! It’s a good thing there is so much, because magnesium has so many uses.
Manufacturing
In its purest form, magnesium is comparable to aluminium. It is strong and lightweight, making it useful for manufacturing automotive components. It’s also used in mobile phones, laptop computers and other electrical devices. Magnesium alloys are becoming more common in the aerospace industry as the need for lightweight fuel-efficient aircraft grows.
Biology – plants
Magnesium is vital for photosynthesis in plants. In fact, a magnesium atom forms the centre of every chlorophyll molecule. Plants use magnesium for other life processes too. Plants get their magnesium from the soil. It is often a macroelement in fertilisers.
Biology – animals
Like plants, animals require magnesium for their cells to function. Animals obtain magnesium from their local environment. Ruminants – cows and to a lesser extent sheep and goats – require magnesium supplements to reduce the risk of milk fever and grass staggers. Milk fever is most often linked with calving and birth – milk and colostrum production removes calcium from the blood. Magnesium helps the animals absorb calcium. Grass staggers occur when animals lack magnesium, often due to milk production and reduced magnesium content in spring pasture growth. Careful pasture and animal management is needed.

Biology - humans
Humans are also very dependent on magnesium. It is one of the seven macrominerals we require to stay healthy. Magnesium is needed for more than 300 biochemical reactions in our bodies. For example, ATP – the main source of energy in our cells – requires a magnesium ion in order to work. Magnesium is also central to maintaining our DNA and RNA structures.
The New Zealand Ministry of Health’s publication Nutrient Reference Values for Australia and New Zealand provides the recommended dietary intake for magnesium. (Note that pregnant and breastfeeding females may have different magnesium requirements.) Green vegetables, nuts and seeds are good sources of dietary magnesium.
Magnesium – recommended dietary intake (RDI) mg/day | |||||
Gender | Age group | RDI | Gender | Age group | RDI |
Male | 1–3 years | 80 mg | Female | 1–3 years | 80 mg |
Male | 4–8 years | 130 mg | Female | 4–8 years | 130 mg |
Male | 9–13 years | 240 mg | Female | 9–13 years | 240 mg |
Male | 14–18 years | 410 mg | Female | 14–18 years | 360 mg |
Male | 19–30 years | 400 mg | Female | 19–30 years | 310 mg |
Male | 31+ years | 420 mg | Female | 31+ years | 320 mg |
Magnesium Atoms
Related content
The Science Learning Hub team has curated a collection of resources related to the periodic table of elements. Log in and make this part of your private collection by clicking on the copy icon. You can then add additional content and notes and make other changes. Registering an account for the Science Learning Hub is easy and free – sign up with your email address or Google account. Look for the Sign in button at the top of each page.
Useful links
Visit the New Zealand Ministry of Health website to download the Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes PDF.

This Stuff article discusses magnesium in human health and which foods we should eat to meet our magnesium requirements.
Visit the Dairy NZ website for more information about magnesium supplementation, milk fever and grass staggers (tetany).
Franklin Vets has information about the symptoms and treatment of milk fever and grass staggers.
Published 29 August 2019Referencing Hub articlesThe magnesium argide ion, MgAr+ is an ion composed of one ionised magnesium atom, Mg+ and an argon atom. It is important in inductively coupled plasma mass spectrometry and in the study of the field around the magnesium ion.[1] The ionization potential of magnesium is lower than the first excitation state of argon, so the positive charge in MgAr+ will reside on the magnesium atom. Neutral MgAr molecules can also exist in an excited state.
Spectrum[edit]
The spectrum of MgAr+ can be observed. It resembles that of Mg+, however some lines are blue shifted and others red shifted. In Mg+ the ground state is termed 2S. A first excited state has a 3s electron moved to the 3p orbital and the state is termed 2P. But because of spin-orbit coupling it is actually split into 2P1/2 and 2P3⁄2 with energy 35,669 and 35,761 cm−1.[1] In comparison the ionic molecule has a ground state called 2Σ+. The corresponding excited state is significantly split into two depending on whether the p orbital of the magnesium is pointing to the argon or is perpendicular. When the electron in the p orbital is perpendicular to the Mg-Ar axis, the argon sees a greater electrostatic force from the magnesium atom and is more tightly bound. This lowers the energy level of what is called the 2Π level. This too is split into 2Π1/2 and 2Π3⁄2. When the excited electron is in line with the argon the state is called 2Σ+ and corresponds only to 2P3⁄2 and so is not split.[1]
The MgAr+ spectrum shows bands, with the first one at 31,396 cm−1, which is redshifted 4300 cm−1 from Mg+. The band is blue degraded. The band consists of a series of doublets. The two lines in the doublet are separated by 75 cm−1, and from one pair to the next one is 270 cm−1. This band is due to A2Π ← X2Σ+.[1]
Properties[edit]
In the ground state the binding energy or MgAr+ is 1281 cm−1 and in the A2Π1/2 state is 5554 cm−1 (3.66 kcal/mol).[1] The A2Π1/2 state has a stronger bond because a p electron overlaps the argon atom less, and thus has less repulsion.[2] The dissociation energy of the ground state ion is 1295 cm−1 (15 kJ/mol).[3]
Magnesium Atomic Radius
The bond length is 2.854 Å for the ground state, and 2.406 Å for the excited state. The 2Π state is predicted to have a radiative lifetime of about 6 nanoseconds.[2]
Neutral molecule[edit]
Unionized MgAr (magnesium argon) can also exist as a van der Waals molecule or temporarily in an excited state termed a Rydberg molecule.[4] The neutral molecule can be formed by evaporating magnesium metal using a laser into argon gas, and then expanding it through a supersonic jet.[5] When evaporated many magnesium atoms are excited into a 3s3p state (from the ground 3s3s). These can then attach an argon atom by way of a three body collision to yield Mg(3s3pπ 3PJ)Ar 3Π. Then this excited state can lose energy via collisions to form Mg(3s3pπ 3PJ)Ar 3Π0+,0−.[6] MgAr is mainly held together with dispersion forces which vary as the inverse sixth power of the separation. The ground state MgAr has electron configuration Mg(3s3s 1S0)Ar 1Σ+.[7] The triplet states with one excited electron include Mg(3s3pπ 3P0)Ar 3Π0+, Mg(3s4s 3S1)Ar 3Σ+, Mg(3s3dδ 3DJ)Ar 3Δ, and Mg(3s4pπ 3PJ)Ar 3Π0+. A singlet single excited electron state is Mg(3s3pπ 1P)Ar 1Π.[7]
The different excited states can be studied by resonance-enhanced two-photon ionization and mass spectroscopy.[6] The absorption spectrum of MgAr shows bands due to electronic transitions combined with vibrational and rotational transitions. The spectrum involving electronic transition in the argon atom and a change in the d orbital of the magnesium, is very complex with 18 different branches[6]
A doubly excited state, where two electrons on the magnesium atom are boosted to 3p sub-orbitals, has a strong binding energy, even higher than in MgAr+.[5] Normally an ion would bond an inert gas atom more strongly, as attraction varies as 1/R4, compared to 1/R6 for a van der Waals molecule, and in an ion, the electron cloud shrinks due to the more positive charge attracting it. However in the doubly excited state both of the magnesium atoms are in p suborbitals, which can be arranged so that electron density is on a line perpendicular to a potential argon atom bond. This allows the two atoms to approach each other closer.[8]
The neutral molecule has cas number 72052-59-6.[9]
| state[7] | electron state | Mg excitation energy cm−1 | MgAr excitation energy cm−1 | bond length Å re | ωe | dissociation energy cm−1 | B0 | Be | αe | D0 centrifugal distortion |
|---|---|---|---|---|---|---|---|---|---|---|
| ground | Mg(3s3s 1S0)Ar 1Σ+ | 0 | 0 | 4.56 | small | |||||
| singlet | Mg(3s3pπ 1P)Ar 1Π | 34770 | 34770 | 3.31 | 175[5] | |||||
| triplet | Mg(3s3pπ 3P0)Ar 3Π0+ | 21850–21911 | 21760 | 3.66 | 102.7 | 1250 | ||||
| [9] | Mg(3s4dσ 3DJ)Ar 3Σ+ | 53462 | 2.88 | 88.2 | 0.1338 | 0.1356 | 0.0037 | 800 | ||
| [9] | Mg(3s4dδ 3DJ)Ar 3Δ | 53063 | 104.1 | 0.1438 | 0.1462 | 0.0037 | 1199 | |||
| [9] | Mg(3s4dπ 3DJ)Ar 3Π0 | 53037 | 99.4 | 1225 | ||||||
| Mg(3s4s 3S1)Ar 3Σ+ | 41197 | 40317 | 2.84 | |||||||
| Mg(3s3dδ 3DJ)Ar 3Δ | 47957 | 46885 | 2.90 | 103.5 | 160[6] | 0.1274 | 0.1291 | 0.0035 | 1140 | |
| Mg(3s3dπ 3DJ)Ar 3Π | 3.27 | 49.05 | 290[6] | 0.1019 | 0.1049 | 0.0061 | 289 | |||
| Mg(3s4pπ 3PJ)Ar 3Π0+ | 47847–47851 | 46663 | 2.84 | 1250[6] | ||||||
| [9] | Mg(3s5pπ 3PJ)Ar 3Π0 | 53049 | 110.1 | 1272 | ||||||
| double | Mg(3p3pπ 3PJ)Ar 3Π0+ | 57812–57873 | 2.41 | 2960[5] |
Solid[edit]
Under pressures over 250 gigapascals, MgAr is predicted to be stable as a solid with either an anti-NiAs or CsCl structure dependent on pressure. Mg2Ar is predicted to be a stable solid with localized electrons in the structure, making it an electride.[10] These pressures are higher than found in the Earth's mantle, but magnesium argides could form minerals in super earths.
Application[edit]
MgAr+ can interfere with determination of copper or zinc isotopes when using inductively coupled plasma mass spectrometry, particularly when using a desolvated plasma. When analysing mineral specimens, magnesium is a common element found in rock matrix. It can react with the argon ions present in the plasma.[11] In analysis of soil, MgAr+ interferes with detection of 65Cu, though common isotopomer has a molecular weight of 64.95 compared to 64.93 for the copper 65 isotope.[12] This is called isobaric interference.
References[edit]
- ^ abcdePilgrim, J. S.; Yeh, C. S.; Berry, K. R.; Duncan, M. A. (1994). 'Photodissociation spectroscopy of Mg+–rare gas complexes'. The Journal of Chemical Physics. 100 (11): 7945. Bibcode:1994JChPh.100.7945P. doi:10.1063/1.466840.
- ^ abBauschlicher, Charles W.; Partridge, Harry (June 1995). 'A study of the X 2Σ+ and A 2Π states of MgAr+ and MgKr+'(PDF). Chemical Physics Letters. 239 (4–6): 241–245. Bibcode:1995CPL...239..241B. doi:10.1016/0009-2614(95)00449-E.
- ^Massick, Steven; Breckenridge, W.H. (August 1996). 'A determination of the ionization threshold for the Mg(3s3p3P0) · Ar(3Π0−) metastable state: The bond energy of MgAr+'. Chemical Physics Letters. 257 (5–6): 465–470. Bibcode:1996CPL...257..465M. doi:10.1016/0009-2614(96)00565-9.
- ^Massick, Steven; Breckenridge, W. H. (8 February 1997). 'Spectroscopic characterization of the 3Δ(4d), 3Π(4d), 3Σ+(4d), and 3Π(5p) Rydberg states of the MgAr van der Waals molecule'. The Journal of Chemical Physics. 106 (6): 2171–2181. Bibcode:1997JChPh.106.2171M. doi:10.1063/1.473673.
- ^ abcdLeung, Allen W.K.; Roberson, Mark; Simons, Jack; Breckenridge, W.H. (August 1996). 'Strong bonding in a doubly excited valence state of a van der Waals molecule'. Chemical Physics Letters. 259 (1–2): 199–203. Bibcode:1996CPL...259..199L. doi:10.1016/0009-2614(96)00723-3.
- ^ abcdefMassick, Steven; Breckenridge, W. H. (8 December 1996). 'Spectroscopic characterization of the excited Mg(3s3d 3DJ)⋅Ar(3Π), Mg(3s3d 2DJ)⋅Ar(3Δ), and Mg(3s4p 3PJ)⋅Ar(3Π) van der Waals states'. The Journal of Chemical Physics. 105 (22): 9719–9732. Bibcode:1996JChPh.105.9719M. doi:10.1063/1.472843.
- ^ abcHald, Kasper; Jørgensen, Poul; Breckenridge, W.H; Jaszuński, Michał (October 2002). 'Calculation of ground and excited state potential energy curves of the MgAr complex using the coupled cluster approximate triples model CC3'. Chemical Physics Letters. 364 (3–4): 402–408. Bibcode:2002CPL...364..402H. doi:10.1016/S0009-2614(02)01339-8.
- ^Massick, Steven; Breckenridge, W. H. (15 May 1996). 'A new class of strongly bound, doubly excited valence states of neutral van der Waals molecules: Mg(3pπ,3pπ 3PJ )⋅Ar(3Σ)'. The Journal of Chemical Physics. 104 (19): 7784–7787. Bibcode:1996JChPh.104.7784M. doi:10.1063/1.471657.
- ^ abcdeHüttner, W. (2012). 'Molecules and Radicals Molecular Constants Diamagnetic Diatomic Molecules'. Landolt-Börnstein Numerical Data and Functional Relationships in Science and Technology. Landolt-Börnstein - Group II Molecules and Radicals. Springer. 29: 53. Bibcode:2012LanB.29A1...25H. doi:10.1007/978-3-540-69954-5_12. ISBN978-3-540-69953-8. ISSN1615-1852.
- ^Miao, Mao-sheng; Wang, Xiao-li; Brgoch, Jakoah; Spera, Frank; Jackson, Matthew G.; Kresse, Georg; Lin, Hai-qing (11 November 2015). 'Anionic Chemistry of Noble Gases: Formation of Mg?NG (NG = Xe, Kr, Ar) Compounds under Pressure'. Journal of the American Chemical Society. 137 (44): 14122–14128. doi:10.1021/jacs.5b08162.
- ^Mason, Thomas F. D.; Weiss, Dominik J.; Horstwood, Matthew; Parrish, Randall R.; Russell, Sara S.; Mullane, Eta; Coles, Barry J. (2004). 'High-precision Cu and Zn isotope analysis by plasma source mass spectrometry'. Journal of Analytical Atomic Spectrometry. 19 (2): 209. doi:10.1039/b306958c.
- ^Duckworth, Douglas C.; Barshick, Christopher M.; Smith, David H. (1993). 'Analysis of soils by glow discharge mass spectrometry'(PDF). Journal of Analytical Atomic Spectrometry. 8 (6): 875. doi:10.1039/JA9930800875.
Extra reading[edit]
- Equipment used to study MgAr+: Hoshino, Hiroshi; Yamakita, Yoshihiro; Okutsu, Kenichi; Suzuki, Yoshitomo; Saito, Masataka; Koyasu, Kiichirou; Ohshimo, Keijiro; Misaizu, Fuminori (June 2015). 'Photofragment imaging from mass-selected ions using a reflectron mass spectrometer I. Development of an apparatus and application to Mg+–Ar complex'. Chemical Physics Letters. 630: 111–115. doi:10.1016/j.cplett.2015.04.033.
- Saidi, Samah; Alharzali, Nissrin; Berriche, Hamid (6 March 2017). 'A combining rule calculation of the ground-state van der Waals potentials of the magnesium rare-gas complexes'. Molecular Physics. 115 (8): 931–941. Bibcode:2017MolPh.115..931S. doi:10.1080/00268976.2017.1292368.
- Bennett, Robert R.; Breckenridge, W. H. (15 January 1992). 'Van der Waals bonding in the lowest electronic states of MgAr, ZnAr, CdAr, and HgAr: Spectroscopic characterization of the b3Π2 and e3Σ+ states of the CdAr molecule'. The Journal of Chemical Physics. 96 (2): 882–890. Bibcode:1992JChPh..96..882B. doi:10.1063/1.462108.
- Gaied, W.; Habli, H.; Oujia, B.; Gadea, F. X. (15 April 2011). 'Theoretical study of the MgAr molecule and its ion Mg+Ar: potential energy curves and spectroscopic constants'. The European Physical Journal D. 62 (3): 371–378. Bibcode:2011EPJD...62..371G. doi:10.1140/epjd/e2011-10572-y.
- Crepin-Gilbert, C.; Tramer, A. (October 1999). 'Photophysics of metal atoms in rare-gas complexes, clusters and matrices'. International Reviews in Physical Chemistry. 18 (4): 485–556. Bibcode:1999IRPC...18..485C. doi:10.1080/014423599229901.
