››Convert grams Glucose to mole
- Atomic Mass Carbon Glucose
- Molecular Weight Of Glucose
- Relative Atomic Mass Of Glucose
- Atomic Mass Of Simple Sugar Glucose
- Molar Mass Of White Sugar
Please enable Javascript to usethe unit converter.
Note you can turn off most ads here:
https://www.convertunits.com/contact/remove-some-ads.php
- However, the experimentally determined molar mass of glucose (180 g/mol) can be used to resolve this dilemma. First, calculate the formula mass, the molar mass of the formula unit, which is the sum of the atomic masses of the elements in the empirical formula multiplied by their respective subscripts.
- Do a quick conversion: 1 moles Glucose = 180.15588 gram using the molecular weight calculator and the molar mass of C6H12O6.
Glucose is a carbohydrate with 6 atoms of carbon, 6 atoms of oxygen and 12 atoms of hydrogen per molecule (C6H12O6). There are 6.02 e23 molecules per mole. So the number of atoms of oxygen in 5 moles of glucose is: 5 X 6.02 e23 = 30.1 e 23 atoms of oxygen.
››More information from the unit converter
How many grams Glucose in 1 mol?The answer is 180.15588.
We assume you are converting between grams Glucose and mole.
You can view more details on each measurement unit:
molecular weight of Glucose ormol
The molecular formula for Glucose is C6H12O6.
The SI base unit for amount of substance is the mole.
1 grams Glucose is equal to 0.0055507486072617 mole.
Note that rounding errors may occur, so always check the results.
Use this page to learn how to convert between grams Glucose and mole.
Type in your own numbers in the form to convert the units!
››Quick conversion chart of grams Glucose to mol
1 grams Glucose to mol = 0.00555 mol
10 grams Glucose to mol = 0.05551 mol
50 grams Glucose to mol = 0.27754 mol
100 grams Glucose to mol = 0.55507 mol
200 grams Glucose to mol = 1.11015 mol
500 grams Glucose to mol = 2.77537 mol
1000 grams Glucose to mol = 5.55075 mol
››Want other units?
You can do the reverse unit conversion frommoles Glucose to grams, or enter other units to convert below:
››Common amount of substance conversions
grams Glucose to centimol
grams Glucose to picomol
grams Glucose to molecule
grams Glucose to nanomol
grams Glucose to micromol
grams Glucose to decimol
grams Glucose to atom
grams Glucose to millimol
grams Glucose to kilomol
››Details on molecular weight calculations
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
››Metric conversions and more
ConvertUnits.com provides an onlineconversion calculator for all types of measurement units.You can find metric conversion tables for SI units, as wellas English units, currency, and other data. Type in unitsymbols, abbreviations, or full names for units of length,area, mass, pressure, and other types. Examples include mm,inch, 100 kg, US fluid ounce, 6'3', 10 stone 4, cubic cm,metres squared, grams, moles, feet per second, and many more!
Lecture 3
Atomic Mass
Covalent compounds are substances formed when non-metallic atoms combine chemically.Most compounds have a fixed atomic composition. The formula of a substance specifies its atomic composition.For example, glucose, a simple sugar, has the formula C6H12O6.This means that 6 carbon atoms are bonded to 12 hydrogen atoms and 6 oxygen atoms, in each molecule of glucose.In theory, we could combine elemental C, O and H in the correct proportions and make a molecule of glucose.Carbon, in its most common state, exists as the solid, graphite.Oxygen exists as the diatomic gas molecule, O2 and hydrogen exists as the diatomic gas, H2.
The smallest detectable speck of graphite would contain about 1 x 1016 atoms of carbon, so we are not going to be able to weigh a single atom.What has been done, instead, is to assign a value to the mass of one carbon atom.By definition, the atomic mass of 12C (carbon with 6 protons and 6 neutrons) has been set as 12 amu (atomic mass units).However, when you look up the atomic mass of carbon in a table, it is shown as 12.01 amu.This is because carbon exists as a number of different isotopes.The most common isotopes of carbon are 12C and 13C (6 protons and 7 neutrons).The amu of 13C has been determined to be 13.00335 amu.It is heavier than 12C because it has an extra neutron and because neutrons have slightly higher masses than protons.12C is more abundant (98.9%) than 13C (1.10%), which is why the listed mass of C is closer to 12 (12.01) than it is to 13.00335.The listed atomic mass represents an average atomic mass of naturally occurring carbon.It was calculated as follows:
average atomic mass of C = (.9890)(12 amu) + (.01100)(13.00335) = 12.01 amu
No real carbon atom has a mass of 12.01 amu.Most carbon atoms have an atomic mass of 12 amu and a few have an atomic mass of 13.00335 amu.The atomic mass of H has been determined to be 1.0079 amu and that of O to be 15.9994 amu.Again, these are average values of the common isotopes of these elements.
Avogadro's Number and Molar Mass
Now we have a measure of the relative masses of the elements.It would be of practical use to somehow convert amu to grams, a unit that we can actually measure.Even the smallest sample of an element contains an enormous number of atoms.Therefore, it would be convenient to have a special unit, which describes a very large number of atoms.This unit is called a mole and is the SI unit of quantity. Paper is sold by reams (500 sheets), eggs are sold by the dozen (12) atoms and molecules are measured in moles.A mole is defined as the number of 12C atoms in exactly 12 grams of 12C.This number has been determined, experimentally, to be 6.022 x 1023 and is called Avogadro's number, NA.
/200257396-001-56a12e8f5f9b58b7d0bcd74b.jpg)
6.022 x 1023 atoms 12C = 12 g 12C
1 atom 12C = 12 amu
6.022 x 1023 atoms = 1 mol atoms
With these conversion factors, we can determine the mass of a single 12C atom.
12 g 12C6.022 x 1023 atoms= 12 g/mol = molar mass of 12C
6.022 x 1023 atoms1 mol
The atomic masses for all of the elements have been tabulated as amu.This same number is also the molar mass, in g/mol, of each element.
So, if we weighed out a 12.01 g sample of carbon, we would have 6.022 x 1023 atoms of carbon in our sample.Looking at our formula for glucose (C6H12O6) we can see that we need twice as many H atoms as C atoms.Our 12.01 g sample of carbon contains 1 mole of carbons atoms.We will, therefore, need 2 moles of H atoms.How many grams of H atoms would this be?We can use the conversion factor relating amu to grams/mol of a substance.
2 mol H1.0079 g H= 2.0158 g H
mol H
How much oxygen would be needed?The formula indicates that for every mole of carbon we need one mole of oxygen.So, we will need 1 mol of O atoms.
1 mol O15.9994 g O= 15.9994 g O
mol O
Our starting materials would weight 12.01g + 2.0158g + 15.9994 g = 30.03 g.
This would also be the mass of the glucose that could be made from these elements.How many moles and molecules of glucose would this be?
The mass of one molecule of glucose, C6H12O6, would be the sum of the atomic masses of its elements:
6 x 12.011 (amu of C) + 12 x 1.0079 (amu of H) + 6 x 15.9994 (amu of O) = 180.157 amu
180.157 amu = 180.157 g/mol
30.03 g glucose1 molglucose= 0.1667 moles of glucose
180.157 g glucose
0.1667 moles glucose6.022 x 1023 molecules glucose = 1.004 x 1023 molecules of glucose
mol glucose
Percent Composition
Another way of describing the composition of a substance is in terms of its percent composition, the percentage, by mass,of the elements in that substance.This is information that is experimentally obtainable, which can be used to derive the empirical formula of a compound, as will be discussed below.For now, lets define what is meant by percent composition by determining the percent composition of sodium nitrite, NaNO2.First, calculate the molar mass:
molar mass =1 mol Na (22.99 g/mol)
+ 1 mol N(14.01 g/mol)
+ 2 mol O(16.00 g/mol)
69.00 g/mol NaNO3
The percent composition of the elements is then:
% Na =22.99 g x 100% = 33.32%
69.00 g
% N =14.01 g x 100% = 20.30%
69.00 g
%O =32.00 g x 100% = 46.38%
69.00 g
It is possible to use this method to determine the formula of an unknown substance.
Analysis of an unknown compound shows the following percent composition:
40.92 % carbon, by weight
4.58 % hydrogen, by weight
54.50% oxygen, by weight
First, assume that you are dealing with some amount of the unknown, say, 100 grams.
40.92 % = 0.4092 x 100 g = 40.92 g of C
4.58 % = 0.058 x 100 g = 4.58 g of H
54.50 % = 0.5450 x 100 g = 54.50 g O
Since atoms combine on a molar basis, not by masses, convert these grams of elements to moles of the different elements.
40.95 g C1 mol C= 3.407 mol C
12.01 g C
4.58 g H1 mol H= 4.54 mol H
1.008 g H
54.5 g O1 mol O= 3.406 mol O
16.00 g O
These numbers indicate the relative number of moles of each of the three elements in the compound.We can now write a formula based on them:
C3.407 H4.54 O3.40
However, whole atoms combine to form molecules, not fractional atoms.So, divide each of these factors by the smallest factor, 3.406.This gives:
CH1.333O
There is still a fractional subscript.Find a factor, which will convert 1.333 to a whole number:
1.333 x 1 = 1.333
1.333 x 2 = 2.666
1.333 x 3 = 4.000
1.333 x 4 = 5.333
Now multiply all of the subscripts by this factor:
C3H4O3
This is called the empirical formula, which tells us the relative numbers of each type of atom in this molecule.This means that the molecule could be:
C3H4O3
C6H8O6
C9H12O9
in other words, (C3H4O3)n
The empirical formula mass (C3H4O3) is:3(12.01 g/mol C)
+ 4(1.008 g/mol H)
+ 3(16.00 g/mol O)
88.06 g/mol
This means that the molecular mass will be some multiple of this value.If we are told that the molecular mass is 176.12 g/mol, we can determine the molecular formula.
(C3H4O3)n = 176.12 g/mol
(C3H4O3)= 88.06 g/mol
(88.06)n = 176.12
n = 2
So, the molecular formula would be C6H8O6.
The most common use of these calculations is to determine the empirical mass for a new or unknown compounds based on the products produced by burning the unknown (combustion reaction).In this reaction, all of the carbon in the compound is converted to CO2, carbon dioxide.All of the hydrogen in the compound is converted to H2O, water.The mass of the CO2 and H2O are carefully measured, and then used to arrive at an empirical formula.
11.5 g of an unknown compound is burned, producing 22.0 g of CO2 and 13.5 g of H2O.What is the empirical formula of the compound?
All of the carbon in the CO2 came from the sample.So, first calculate the number of moles of carbon in the 22.0 g of CO2.
22.0 g CO2mol CO21 molC= 0.500 moles C
44.01 g CO21 mol CO2
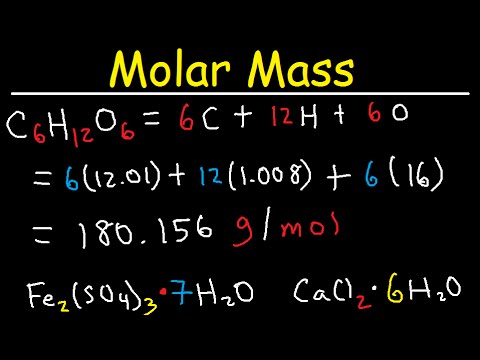
All of the hydrogen in the water came from the unknown, so calculate the number of moles of hydrogen in the 13.5 g of H2O.
13.5 g H2O1 mol H2O2 mol H= 1.50 moles H
18.03 g H2O1 mol H2O
We also have to determine if any of the oxygen in the CO2 and H2O came from the unknown, or whether it was environmental oxygen used in the combustion reaction.To determine this, we need to compare the mass of the unknown to the mass of the hydrogen and carbon that we know came from the unknown.
massunknown=mass hydrogen + masscarbon + massoxygen
mass hydrogen = 1.50 mol H1.0079 g H =1.51 g H
Atomic Mass Carbon Glucose
1 mol H
masscarbon = 0.500 mol O12.011 g C = 6.00 g O
Molecular Weight Of Glucose
1 mol C
massunknown = 11.5 g = 1.51 g + 6.00 g + massoxygen
massoxygen = 4.0 g
So, 4.0 g of the oxygen must have come from the unknown.Convert this to moles of oxygen
4.0 g O1 mol O=0.25 moles O
15.9994 g O
Relative Atomic Mass Of Glucose
Now we can determine the empirical formula of the unknown.First, substitute the calculated numbers of moles into the formula:
C0.5H1.5O0.25
Atomic Mass Of Simple Sugar Glucose
Divide by the smallest fraction (0.25), since these are non-integer subscripts.This gives:
Molar Mass Of White Sugar

C2H6O
In order to determine the molecular formula, we would need to know the molar mass to determine if the unknown is C2H6O or C4H12O2 or C6H18O3.
